
Fructose and ATP Trapping - Contributing to Obesity?
It’s human nature to look for something (or someone) to blame whenever we’re faced with a crisis. That “thing” in regards to the current epidemic of obesity, type 2 diabetes, and metabolic syndrome is sugar (more specifically, refined sugar and high-fructose corn syrup).
Why is sugar in the crosshairs?
Well, it tastes sweet, is void of fiber (as well as any other micronutrient), and it’s crammed into just about every processed food you’ll buy at the store -- which is the main type of food the average American consumes.
Related - Carb 10: Your Optimal Training Carbohydrate Source
Note: These foods are also generally low in fiber and micronutrients, too.
As a result, we tend to consume a lot of sugar as a population, but interestingly sugar consumption has actually been trending downward over the past few years.
So, why is it then that we’re still faced with climbing numbers of people who are getting sick, fat, and doomed to a life of prescription medications?
Well, it’s not entirely sugar’s fault. You don’t get fat from simply eating sugar.
You get sick, fat, and nearly dead from consuming an excessive amount of calories, which usually contain lots of sugar, fat, and salt, along with a severe lack of physical activity.
The next question is why are these foods so easy to eat in excess?
Well, the simple answer is that they’ve been engineered to have the perfect ratio of sugar, fat, salt, and in some instances crunch, that tickle our taste buds and neurological pleasure receptors to keep us coming back for more.
And, for the average person, that answer is good enough.
But, I’m not the average person, and I like to dig deep... Real deep.
I want to know what it is about each of these compounds (sugar, fat, and salt) that make them so highly craveable and keep us eating, and eating, and eating, even though we’ve had way past our fill by calorie need standards.
Today, we’re going to focus on the role (if any) that sugar plays in hunger, more specifically, a particular type of sugar -- fructose.
Fructose 101
Fructose is a monosaccharide (simple sugar) primarily found in fruits and honey, hence it’s more common name fruit sugar.
There are three primary sources of fructose in the modern human diet, including:
- Fruit
- Refined sugar (which is equal parts glucose + fructose)
- High-fructose corn syrup (HFCS)
Now, many people instantly assume the fetal position when hearing the phrase high-fructose corn syrup, due to a lot of the negative press it has received in recent years. In fact, many people single out high-fructose corn syrup as the root cause of the rise in obesity and type 2 diabetes.
So, what exactly is high-fructose corn syrup?
It’s a common sweetener used in many processed foods, and it was introduced into the market in the late 1960's. Its popularity and use in processed foods have only increased since then due to the fact that it is easier and cheaper to produce than sucrose (refined sugar).
This is due to the fact that corn is more abundant and readily available in the USA than sugar cane and sugar beets, which are the crops used to derive sucrose.
Now, the reason a lot of people think that high-fructose corn syrup is bad is due to the fact that it’s processed, is genetically modified, and contains “high” amounts of fructose.
For what it’s worth, everything is genetically modified to one extent or another, whether its directly modifying the DNA sequence or modifying the expression of those DNA sequences (see the field epigenetics for more info). But, that’s a story for another day.
Back to fructose and why high-fructose is so “bad.”
When you step outside of the fear-mongering blogosphere, you’ll see that “high” fructose corn syrup isn’t all that different from regular sucrose, at least from a sugar composition standpoint.
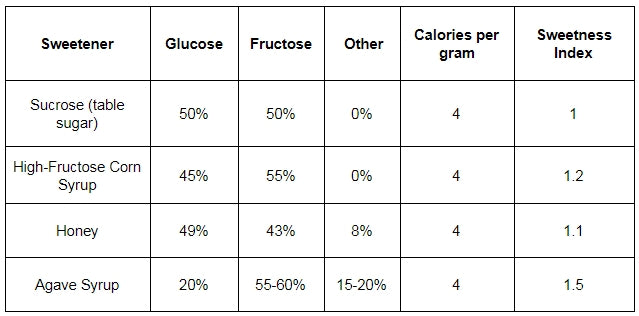 As you can see, there’s only a marginal 5% difference in the sugar ratios between the two.
As you can see, there’s only a marginal 5% difference in the sugar ratios between the two.
So, you mean to tell me that as a populace, we’re all getting sick over a 5% difference?
I’d be hard pressed to believe that.
And for what it’s worth, agave syrup, which is considered by many of the “health gurus” around the internet to be more “natural” and “wholesome” actually has a higher percentage of fructose and is more sweet gram for gram than high-fructose corn syrup.
Yet, you don’t see news reports blasting day and night blaming agave syrup for the current health crisis.
Interestingly, while we’re quick to blame high-fructose corn syrup for the rising rates of obesity, type 2 diabetes and metabolic syndrome, the population’s consumption of the sweet syrup has steadily been dropping since its peak in the early 2000s. [1]
And, researchers have also found that there is “no good human evidence that fructose, when consumed in isocaloric amounts, causes more liver fat accumulation than other energy-dense nutrients.” [3]

So, if we’re consuming less fructose on average than we were roughly 20 years ago, why are waistlines, health care costs, and insulin prescriptions still expanding?
Well, it might not have to do with the amount of fructose we’re consuming, but the fact that we’re consuming fructose in general.
Maybe fructose manipulates the hunger and energy signals in our body, meaning that when we eat fructose or foods high in it, we might actually end up hungrier than we initially were, even though we’ve consumed a significant amount of calories and should be sated.
In other words, maybe it’s not fructose that is directly making us fat, per se, but maybe it’s messing with our hunger and satiety signals, causing us to eat more calories, and it’s these excess calories, that are causing us to get fatter and sicker.
And in fact, some animal studies have noted that ingesting similar amounts of glucose and fructose can have differing effects on metabolism and appetite.
But, I think we’d all agree that human physiology isn’t quite the same as that of a lab rat.
So, what do the human studies have to say about fructose vs glucose intake and appetite regulation?
Unsurprisingly, they show the same thing. When subjects are given drinks sweetened with fructose versus one sweetened with an equivalent amount of glucose, they have a greater appetite and desire for food compared to the glucose-sweetened beverage. [4,5]
Interestingly, human subjects consuming fructose-sweetened foods and drinks are also more willing to give up monetary rewards in order to get high-calorie “food rewards” faster too! [5]
But, why is this?
Both glucose and fructose supply the same amount of energy (4 calories per gram), so shouldn’t they both provide similar levels of satiety?
Not quite.
And, in order to understand why, we need to discuss the differences between the two sugars are metabolized in the body.
How is Fructose Metabolized?
Following ingestion, free fructose enters into the endothelial cell of the intestinal wall via GLUT 5 (glucose transporter 5) and is transported out of the intestinal lumen via GLUT 2 transporter, which is an insulin-independent transporter of glucose. Keep this in mind going forward.
Glucose is transported into the endothelial cells of the intestinal lumen by SGLT1 and transported out by GLUT2.
If fructose is part of a larger sugar (such as sucrose), the enzyme sucrase cleaves the two molecules apart, and from there they can be absorbed into the intestinal lumen.
Now, once fructose is shuttled into circulation, it’s sent first to the liver, as it is the primary site for fructose metabolism in the body.
The easiest way to show the differences between the metabolization of both sugars is with a picture: [3]
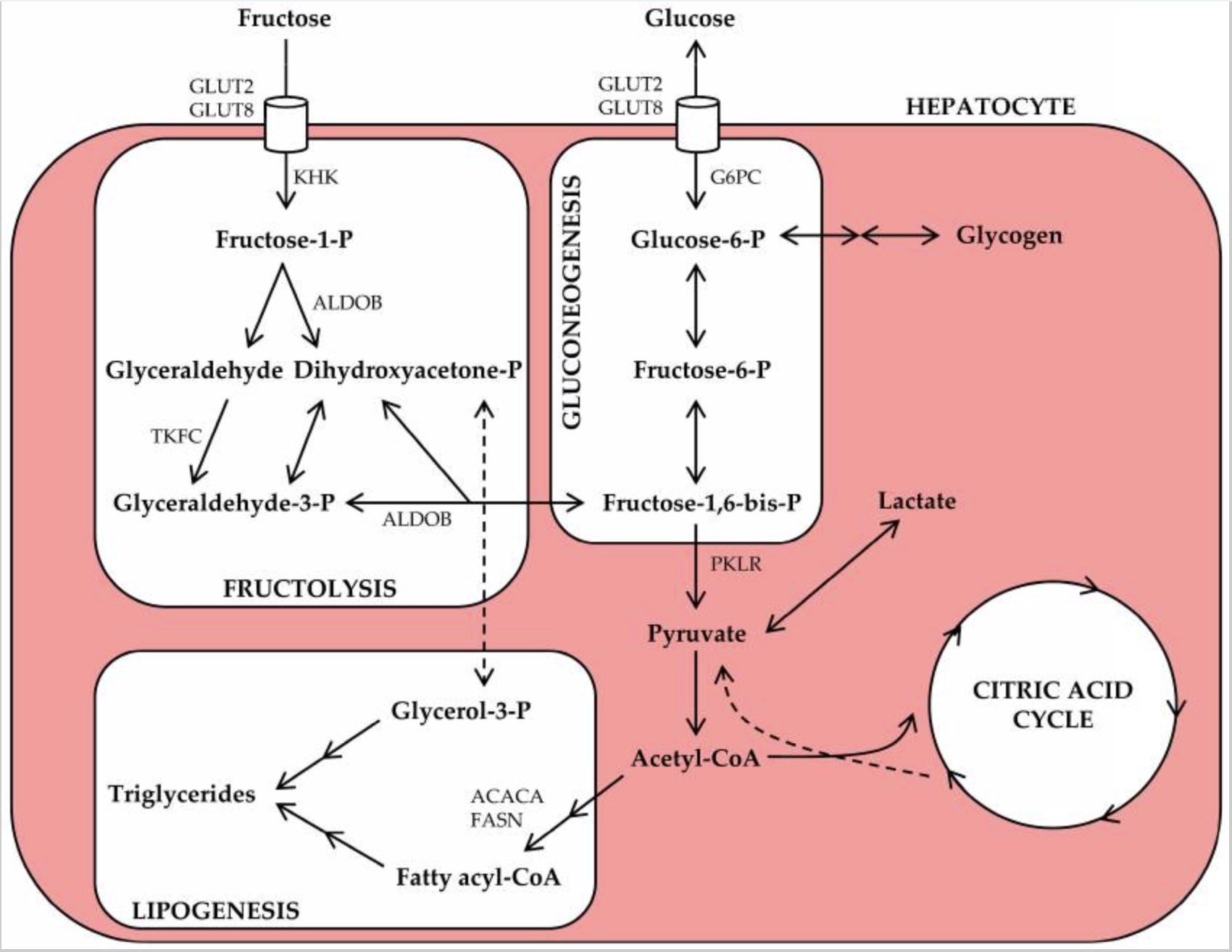 What’s not so clear from this picture is the fact that unlike glucose, fructose requires ATP for its metabolism.
What’s not so clear from this picture is the fact that unlike glucose, fructose requires ATP for its metabolism.
The first part of fructose metabolism (a process called fructolysis) is catalyzed when fructose is phosphorylated with the enzyme fructokinase. In order for fructose to be phosphorylated, a molecule of ATP has to be “burned.”
This process is irreversible and results in the molecule Fructose-1-Phosphate.
Fructose-1-Phosphate is then cleaved into glyceraldehyde and dihydroxyacetone-phosphate by the enzyme aldolase B. This gives us glyceraldehyde-3-phosphate which can then go through the rest of the regular glycolytic pathway (the pathway that metabolizes glucose).
Fructose metabolism also bypasses the phospho-fructose-kinase 1 (PFK-1) enzymatic pathway, which is important as the PFK-1 enzyme is one of the most tightly regulated enzymes in the body.
This enzyme is activated by “energy depleted” states in the body (AMP, Fructose-2,6-bisphosphate) and inactivated by “energy rich” states (ATP, Citrate, and H+).
The fact that fructose skips this step is critically important as it essentially means that fructose metabolism can basically go unchecked.
As a result of this “lax” regulation of fructose metabolism, we end up with a lot of acetyl-CoA -- more than we generally need at a given time. This means that any unused acetyl-CoA is turned into fatty acids, which eventually forms triglycerides. Not all of this fat can escape the liver which eventually leads to non-alcoholic fatty liver disease (NAFLD).
So, there’s problem #1 with a large intake of fructose.
But, that doesn’t answer the reason why research finds that we tend to be hungrier when consuming foods higher in fructose compared to glucose.
For that, we need to turn to fructose’s effects on the brain.
Fructose, Hormones, ATP Trapping, and Appetite Regulation
While most people think that the liver is the only place where fructose can be used, research has actually shown that the hypothalamus possesses both Glut5 and ketohexokinase mRNA, the requisite “cellular machinery” to metabolize fructose.
Why is this noteworthy?
Well, the hypothalamus is tasked with keeping the body in a state of homeostasis, as such it is responsible for sensing how much “fuel” we have (ATP) as well as regulating many hormones in the body, including the ones that modulate hunger and appetite signals.
Basically, fructose can cross the blood-brain barrier and be used by neurons as a source of fuel. [9,10,11,12]
To determine the reason why individuals feel more hungry after consuming food and drink higher in fructose than an equivalent amount of glucose, they collected blood samples and took scans of the individual's brains, the hypothalamus in particular. [7]
Essentially what they found was that our bodies appear to “recognize” the glucose-fueled calories we’ve eaten, responding with an increase in blood glucose levels and insulin.
Insulin itself is a satiety-promoting hormone in the body. [14]
Researchers also noted that neurological activity in the hypothalamus slowed following ingestion of the glucose-containing drink, indicating that appetite was lowered.
However, following the fructose drink, insulin levels barely increased, and the hypothalamus remained active, providing two-different ways in which fructose “sneaks” around the appetite-regulating mechanisms of the body.
Interestingly, levels of ghrelin (the “hunger” hormone) and leptin (the “satiety” hormone) were unchanged after consuming either the glucose-rich drink or the fructose-rich one.
Researchers suggest this may be due to the fact that their observational interval was too brief as it can take up to 6 hours after glucose ingestion for leptin levels to rise. [5]
Regardless, the takeaway here is that when study participants drank the glucose-rich drink their bodies felt fuller and more sated than the fructose-sweetened one. [5,13]
This study highlights the phenomenon known as “ATP trapping” whereby the body should have plenty of energy from sugar (fructose), but since the proper signals are not reaching the brain, our bodies are under the impression that we are lacking energy, thereby driving us to consume more calories in an effort to correct this perceived energy deficiency.
Remember, fructose metabolism bypasses the rate-limiting step in glycolysis (PFK-1), which is critical to the regulation of ATP and energy intake. Since fructose metabolism bypasses this, fructolysis can rapidly deplete ATP in the hypothalamus (as well as the liver).
And, as ATP levels fall, AMP (adenosine monophosphate) levels rise, activating AMPK, which leads to phosphorylation and inhibition of ACC (acetyl-CoA carboxylase). This inhibition of ACC leads to decreased malonyl-CoA levels in the hypothalamus.
Malonyl-CoA is an important energy regulator level in the body, and when it is depressed (or inhibited), food intake increases. [18]
Now, here’s the catch, while glucose and fructose utilize the same signaling pathway to regulate food intake they have different effects on hypothalamic malonyl-CoA, which is part of the reason why we feel hungrier when consuming something sweetened with fructose than an isocaloric food that uses glucose.
Basically, since fructose doesn’t garner the same response from the body’s satiety-signaling mechanisms (insulin, leptin, GLP-1, etc), we continue to keep eating food in the hopes of activating these mechanisms.
Essentially, your brain thinks you’re starving, when in reality you’ve been force-feeding it calories way past the point of needing it.
Inevitably, this leads to the overconsumption of calories, typically from more of the same hyper-processed foods that started this whole ordeal in the first place.
This “trapping” phenomenon has been highlighted in some animal studies where rats were infused with an analog of fructose called 2,5-anhydro-D-mannitol. [17]
Researchers observed that the fructose analog was rapidly phosphorylated in the liver, “trapping” hepatic phosphate and decreasing ATP, which led to increased food intake. [6]
The way the researchers corrected this was one of two ways:
- Direct treatment of capsaicin to the vagus nerve, or
- “Selective vagotomy” -- surgical removal of a piece of the vagus nerve.
FYI, the vagus nerve is the longest and most complex of the 12 cranial nerves we have and is at the heart of the gut-brain axis.
However, aside from these hormones and neurological signals that tell us whether we are full or not, it appears that the liver itself plays a key role in hunger and satiety.
Earlier studies found that the brain can directly communicate to the liver to regulate glucose production via hypothalamic nutrient sensing and vagal nerve action. And, newer research appears that the flip side is true -- the liver communicates to the brain and transmits signals through (you guessed it) the vagus nerve.
Now, beyond the liver-hypothalamus link and the release of hunger/satiety hormones, a number of other factors influence our desire to eat as well as our feeling of fullness and satisfaction from food including psychological and behavioral responses to food as well as “cephalic-phase responses”, which are the signals generated in our bodies by the sight and smell of food.
All of these work in conjunction with the aforementioned factors to influence hunger, appetite, and satiety.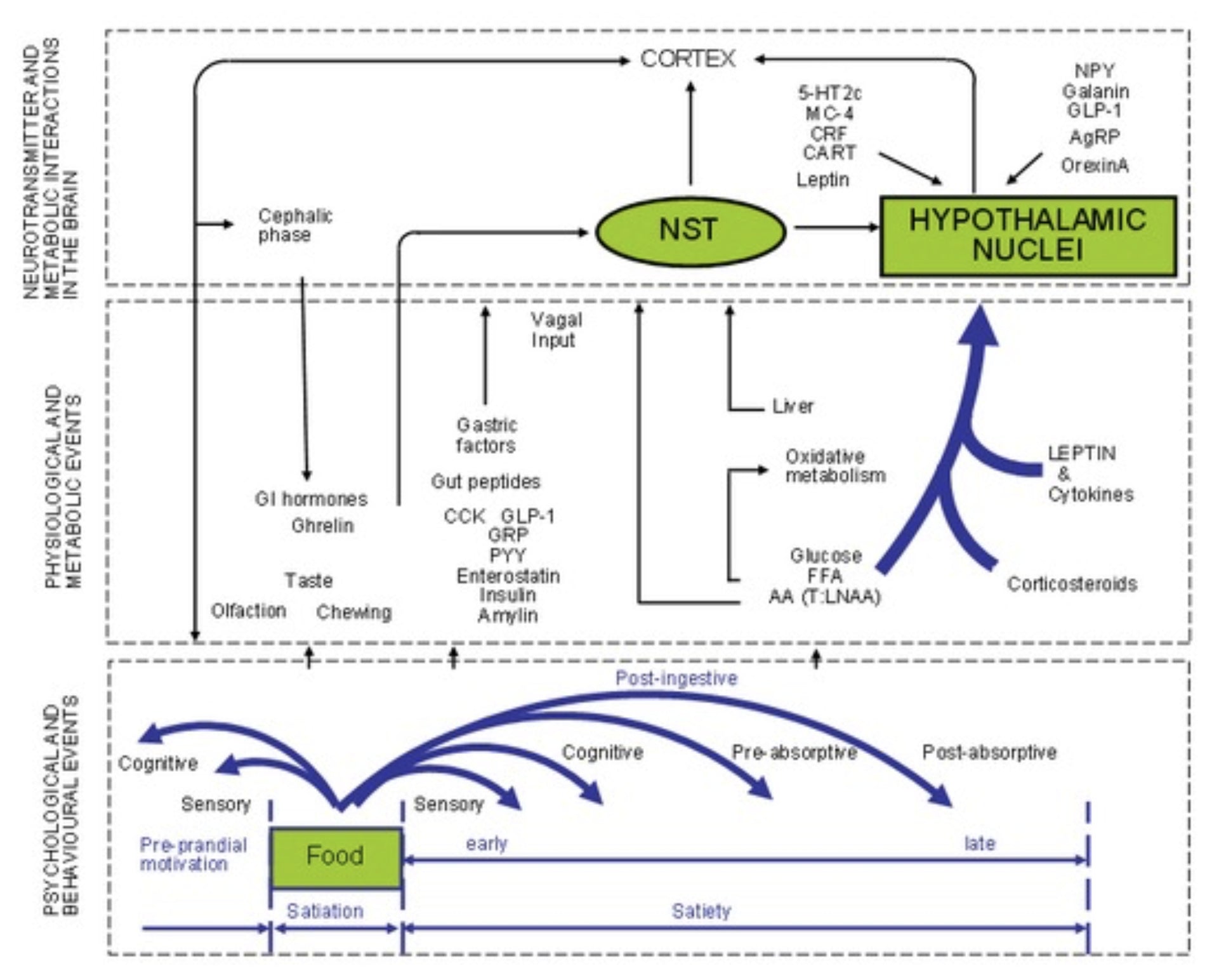
Figure 1: Diagram showing the expression of appetite as the relationship between three levels of operations: the behavioral pattern, peripheral physiology and metabolism, and brain activity. PVN, paraventricular nucleus; NST, nucleus of the tractus solitarius; CCK, cholecystokinin; FFA, free fatty acids; T: LNAA, tryptophan: large neutral amino acids (See (4)for detailed diagram). [2]
And while, fructose ingestion represents just one piece of this intricate puzzle, making it a considerable chunk of your diet isn’t doing you any favors for keeping energy intake within reason.
So, What About Fruit?
Now, now doubt you’re wondering where fruit comes into the picture.
After all, fruit contains (a small amount of) fructose. Does that mean our “apple a day” is actually making us more hungry?
Not quite.
While fruit does contain fructose, that fructose in fruit is surrounded by a fiber matrix, which slows down the digestion. Fruit also has a high water content, which combined with fiber, help keeps us full.
And, the fructose in fruit is also packed alongside essential vitamins, minerals, and polyphenols, which are known to support health.
Products containing refined sugar and/or high-fructose corn syrup rarely come packed with fiber or have a high water content or a rich supply of essential vitamins and minerals.
Plus, to consume enough fructose from fruit to match what can be achieved by a few super-sized sodas were verge on your consuming pounds of fruits, not the daily apple or bowl of blueberries you have for breakfast.
In other words, you can eat fruit (even daily) and not worry about it screwing with your hunger signals, ATP depletion, or anything else.
Takeaway
Like most things, fructose (in whatever) form isn’t inherently bad, and it’s not going to make you fat or diabetic.
It is the excessive consumption of it in the presence of a gross calorie surplus that sets humans on the path to illness, disease, and death.
Now, that being said, most foods and drinks using added fructose (in the form of refined sugar or high-fructose corn syrup) contain massive amounts of calories, and, especially in the case of sodas, are incredibly easy to overconsume without signaling the body of their true calorie payload.
And, given the points we discussed above regarding fructose and satiety, if you’re looking to keep your diet (and waistline) in check, you’d be well served to limit your fructose intake to that which is naturally occurring in fruit.

Leave a comment